
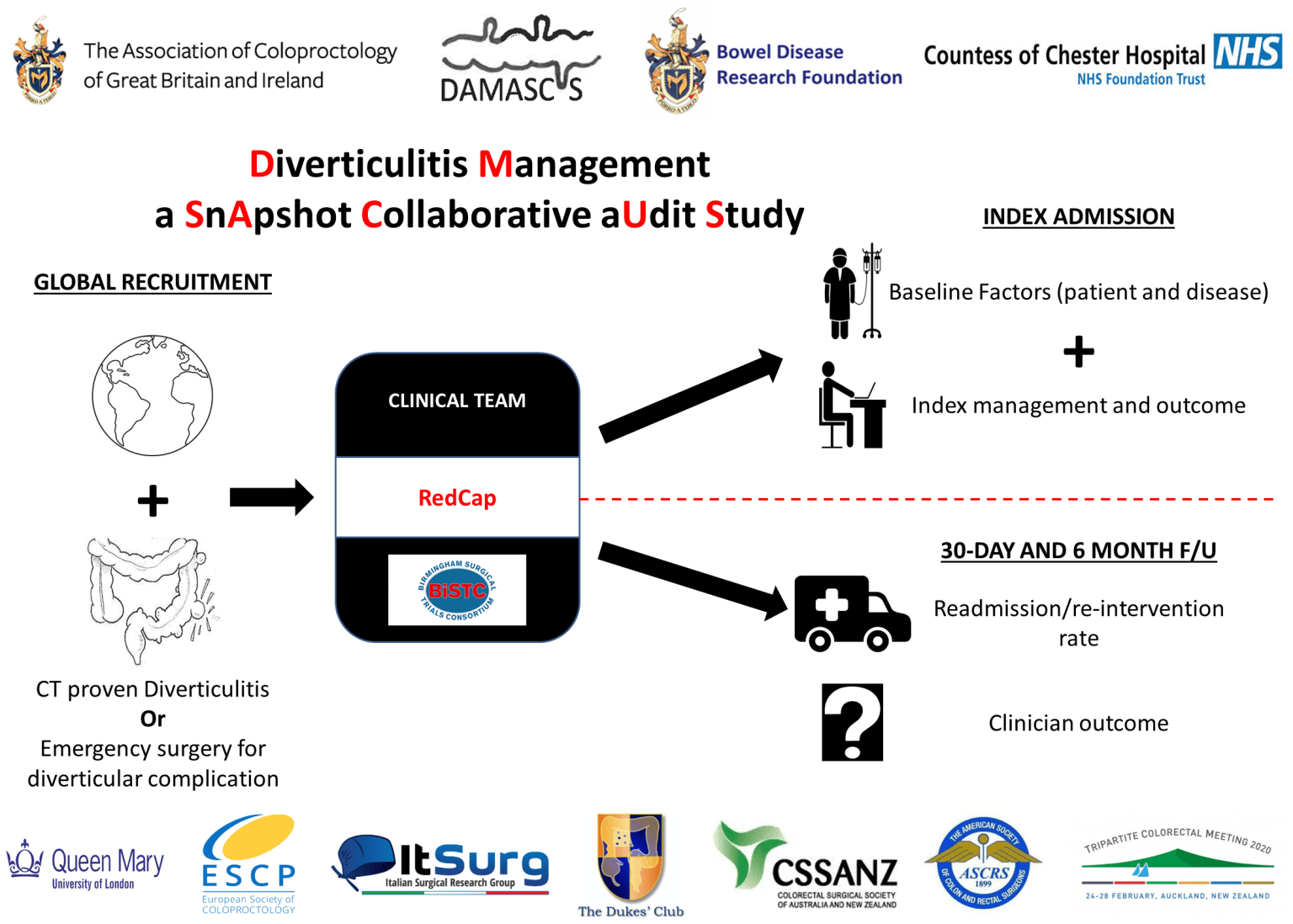
Diverticulitis Management: A Snapshot Collaborative Audit Study
DAMASCUS is an international, prospective study aiming to investigate the international variability in the presentation and index management of acute diverticulitis, and whether there is an association between index management and short and medium-term clinical outcomes including the readmission or re-intervention rates over the subsequent 6 months. DAMASCUS is a trainee-led study with local consultant support.
Steps required to recruit to DAMASCUS
-
DAMASCUS patient inclusion window open
The recruitment window for DAMASCUS opened on 1st October but we recognise that as a global study a single start date that suits everyone is impossible in the current climate so there is a rolling start date from 1st October. Once you have permission at your hospital you should start tracking eligible patients and recording their data for a minimum of 6 consecutive months, followed by six months follow up. We have now closed recruiting new centres to the study. Participating centres will continue patients recruitment until the end of August 2021.
-
Data collection
For centres that are already uploading data to the study database remember to record the patient REDCap ID against a hospital identifier and maintain this link information securely at your hospital site.
If you do not have a login to the study database in REDCap you may still start tracking and recording the details of eligible patients on paper if you have permission from your hospital. Hard copy CRFs are attached to this email.
-
Our team details have changed / I’ve moved hospitals and would like to sign up a new centre to take part in DAMASCUS
Given the 6-month follow up of this audit, we appreciate that data collection will likely cross over training rotations. Please contact us through this link to let us know about any changes to your team throughout the study (each team can have up to 10 collaborators).
Country leads for DAMASCUS
Country leads will coordinate the opening of recruiting sites in a single country, specifically, they will support local investigators by:
- Helping with local country-specific ethical applications/reviews
- The local translation of study documentation
- Coordinate communication with CI’s and the study centre
Requirements expected for the Country leads:
Successful country leads will:
- Open at least 3 centres in their country
- Each site will recruit at least 3 patients over the duration of the study i.e. one patient recruited every 2 months
The country leads will be acknowledged separately in presentation/publications. Hierarchically they will sit as follows:
- Study management group.
- Writing group.
- The country leads group (1 lead per country, more than 1 will be considered in countries that have a high volume of units i.e. over 12.
- Local investigators (up to 10 collaborators per site) and site lead investigators (denoted by an asterix*), organised by site – in alphabetical order, by country.
Details of country leads will soon be posted upon the Dukes Club website once a finalised list has been compiled.
Important links
COVID updates to eCRF
Given that some recruitment will take place during COVID surges we have added a few questions to the CRF that briefly cover whether COVID status (patient or unit) influence the management of patients at any point.
Given that some recruitment will take place during COVID surges we have added a few questions to the CRF that briefly cover whether COVID status (patient or unit) influence the management of patients at any point.
Download DAMASCUS protocol
Frequently Asked Questions
Who is already involved?
There are over 110 sites signed up across the world. Please contact us if your hospital has signed up and you wish to join the team at your hospital.
Sign me up!
The study has now closed for new centres.
Do I need to get ethical approval to join the study?
Ethical approval will be sought within each host nation according to local, state or national policy.
In the UK, this audit is not considered to be research and as such sites will be able to participate once local clinical audit approval is in place.
You can download Mount Sinai’s IRB approved informed consent form (ICF) from here IRB-Approved-ICF
Which guidelines I should use for the audit proposal?
The EAES and SAGES 2018 consensus conference on acute diverticulitis management: evidence-based recommendations for clinical practice can be found here to be used for local audit registration.
Who do I contact to get involved?
For more information, please contact us at Damascus.study@gmail.com.
If you need to present the study to your department, you may use this presentation if you wish.